一种含1,3,4-噁二唑的喹唑啉-4(3H)-酮类衍生物、制备方法及应用与流程
 2021-02-02 16:02:16|
2021-02-02 16:02:16| 339|
339| 起点商标网
起点商标网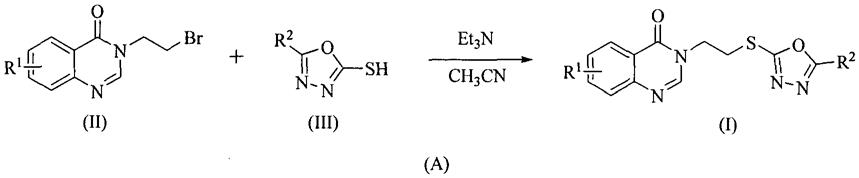
一种含1,3,4-噁二唑的喹唑啉-4(3h)-酮类衍生物、制备方法及应用
技术领域
[0001]
本发明涉及农药领域,具体来说涉及一种含1,3,4-噁二唑的喹唑啉-4(3h)-酮类衍生物,同时涉及该类衍生物的制备方法,还涉及该类衍生物在防治植物病原微生物方面的应用。
背景技术:
[0002]
每年因植物病原微生物侵染农业经济作物而造成的经济损失巨大。当前,化学防治在治理植物病原微生物的流行爆发方面发挥有重要作用。然而,随着杀菌剂的持续性使用,部分杀菌剂品种逐渐显现出对非靶标生物毒性较大、作用靶点相对单一和对环境污染严重等问题,限制了其在农业生产上的应用。因此,研制具有高效、对靶标生物专一性好和环境友好型的新型杀菌剂对保障农业增产稳产和粮食安全具有重要的作用和意义。
[0003]
喹唑啉-4(3h)-酮作为一种重要的天然活性亚结构片段被广泛应用在杀菌剂的创制工作中。例如,阿巴康唑和氟喹唑即被开发为抑制病原真菌几丁质生物合成的广谱抑菌剂。近年来的研究表明,在喹唑啉-4(3h)-酮结构的3位引入希夫碱(j.agric.food chem.2013,61:9575-9582;eur.j.med.chem.2014,77:65-74;chem.pap.2016,70:1521-1528.)、1,2,4-三唑(chem.pap.2016,70:983-993;chin.j.org.chem.2018,38:531-538.)、1,2,4-三唑并[1,5-a]嘧啶(mol.divers.2018,22:1-10.)和1,2,4-三唑并[3,4-b][1,3,4]噻二唑(j.saudi.chem.soc.2018,22:101-109.)等含氮结构后所得化合物对植物病原菌具有良好的抑制作用。
[0004]
另一方面,含有1,3,4-噁二唑活性基团的化合物因具有杀虫、抑真菌、抑细菌和抗病毒等较为广谱的农业生物活性而逐渐受到农药创制工作者的青眯。近年来的研究表明,1,3,4-噁二唑衍生物对烟草青枯菌(j.agric.food chem.2012,60:1036-1041.)、番茄青枯菌(bioorg.med.chem.lett.2014,24:1677-1680.)、柑橘溃疡病菌(mol.diver.2018,22:791-802)和水稻白叶枯菌(chem.pap.2017,71:1953-1960.)、对黄瓜灰霉病菌(chin.j.pestic.sci.2004,6(3):71-73)、水稻纹枯病菌、小麦赤霉病菌(chin.j.org.chem.2014,34:1447-1451)等植物病原细菌与真菌表现有良好的抑制效果。
[0005]
综上,喹唑啉-4(3h)-酮类化合物和1,3,4-噁二唑类化合物对病原真菌和细菌具有广谱的生物活性,对发现新型农药先导化合物具有重要意义。鉴于此,为创制对植物病原微生物具有高效广谱抑制活性的新型化合物,本发明将喹唑啉-4(3h)-酮与1,3,4-噁二唑结构通过硫醚结构片段进行有机结合,设计并合成了一种对植物病原微生物具有明显抑制作用的新型含1,3,4-噁二唑的喹唑啉-4(3h)-酮类衍生物。
技术实现要素:
[0006]
本发明的目的在于,提供一种含1,3,4-噁二唑的喹唑啉-4(3h)-酮类衍生物。
[0007]
本发明的另一目的在于提供含一种含1,3,4-噁二唑的喹唑啉-4(3h)-酮类衍生物
的制备方法。
[0008]
本发明的第3个目的在于提供上述衍生物的用途。
[0009]
本发明的第一方面提供了一种具有通式(i)所示结构的一种含1,3,4-噁二唑的喹唑啉-4(3h)-酮类衍生物,
[0010][0011]
在式(i)中,所述各个基团具有如下所述的定义:
[0012]
r
1
选自下列1~4个基团:氢原子、卤素、c
1
~c
6
烷基、含1~3个卤素的c
1
~c
6
烷基、c
1
~c
6
烷氧基、硝基;
[0013]
r
2
选自苯基、取代苯基、苯基甲基、取代苯基甲基、苯氧基甲基、取代苯氧基甲基、苯硫基甲基、取代苯硫基甲基、芳香杂环基,所述的取代苯基是指其苯环上含有1~5个下列基团:卤素、c
1
~c
6
烷基、含1-3个卤素的c
1
~c
6
烷基、c
1
~c
6
烷氧基、硝基,所述的芳香杂环是指含有1~3个o、n、s杂原子的五元或六元芳香杂环;
[0014]
以上基团的定义中提及的卤素选自f、cl、br、i。
[0015]
在式(i)中,所述各个基团具有如下所述的优选定义:
[0016]
r
1
选自下列1~2个基团:氢原子、卤素、c
1
~c
6
烷基、c
1
~c
6
烷氧基;
[0017]
r
2
选自取代苯基、取代苯基甲基、苯氧基甲基、取代苯氧基甲基、芳香杂环基,所述的取代苯基是指其苯环上含有1~3个下列基团:卤素、c
1
~c
6
烷基、c
1
~c
6
烷氧基、硝基,所述的芳香杂环是指含有1个o、n、s杂原子的五元芳香杂环;
[0018]
以上基团的定义中提及的卤素选自f、cl、br。
[0019]
在式(i)中,所述各个基团具有如下所述的进一步优选定义:
[0020]
r
1
选自下列1~2个基团:氢原子、cl、甲基、甲氧基;
[0021]
r
2
选自取代苯基、取代苯基甲基、苯氧基甲基、取代苯氧基甲基、呋喃基,所述的取代苯基是指其苯环上含有1~3个下列基团:f、cl、甲基、甲氧基、硝基。
[0022]
在式(i)中,所述各个基团具有如表1中所述的更进一步优选定义:
[0023]
r
1
选自氢原子、6-氯、8-甲基、6,7-二甲氧基;
[0024]
r
2
选自4-氯苯基、4-硝基苯基、3,4,5-三甲氧基苯基、2-甲基苯甲基、4-氯苯甲基、4-氟苯甲基、苯氧基甲基、4-氯苯氧基甲基、4-氟苯氧基甲基、呋喃-2-基。
[0025]
在式(i)中,所述各个基团具有如表1中所述的特别优选定义:
[0026]
表1 化合物i的名称及结构
[0027]
[0028]
[0029][0030]
本发明的第二个方面提供了如通式(a)所示的一种含1,3,4-噁二唑的喹唑啉-4(3h)-酮类衍生物(i)的制备方法,在三乙胺(et3n)存在下,含取代基r
1
的3-(2-溴乙基)喹唑啉-4(3h)-酮(ii)与含取代基r
2
的1,3,4-噁二唑-2-硫醇(iii)反应,生成一种含1,3,4-噁二唑的喹唑啉-4(3h)-酮类衍生物(i):
[0031][0032]
其中在上述各结构式中:
[0033]
r
1
、r
2
均具有如前所述的相应基团的定义。
[0034]
本发明的第三个方面提供了式(i)所示的一种含1,3,4-噁二唑的喹唑啉-4(3h)-酮类衍生物的应用,该类衍生物对植物病原真菌和细菌具有显著的抑制活性,可应用于防治植物真菌、植物细菌病害。
[0035]
本发明式(i)的一种含1,3,4-噁二唑的喹唑啉-4(3h)-酮类衍生物适合于抑制水稻白叶枯病菌、水稻纹枯病菌和小麦赤霉病菌,适合用于防治水稻纹枯病、小麦赤霉病、水稻白叶枯病。
[0036]
有益效果:
[0037]
本发明与现有技术相比,具有明显的有益效果,从以上技术方案可知:本发明通过硫醚结构片段将1,3,4-噁二唑基团引入喹唑啉-4(3h)-酮的结构中,设计合成了一系列含1,3,4-噁二唑的喹唑啉-4(3h)-酮类衍生物(i),该类化合物的结构具有新颖性;将该类化合物应用于抗植物病原细菌和真菌方面的研究,发现此类化合物在抗植物病原细菌和真菌方面拥有突出的抑制活性,体现出本技术方案的显著进步;其中部分化合物对水稻白叶枯病菌、水稻纹枯病菌和小麦赤霉病菌的抑制活性超过对照药剂噻菌铜或恶霉灵,体现了明显的应用性。
具体实施方式
[0038]
实施例一:3-(2-((5-(4-氟苄基)-1,3,4-噁二唑-2-基)硫基)乙基)喹唑啉-4(3h)-酮(ia)的合成:
[0039]
将3-(2-溴乙基)喹唑啉-4(3h)-酮(iia)(2.50mmol)、5-(4-氟苄基)-1,3,4-噁二唑-2-硫醇(iiia)(2.50mmol)、三乙胺(7.50mmol)和乙腈(30ml)加入到50ml三口瓶中,体系加热至回流后继续反应约4小时,随后冷却至室温,减压除溶剂,柱层析(v
石油醚
∶v
乙酸乙酯
=1∶2),即得化合物3-(2-((5-(4-氟苄基)-1,3,4-噁二唑-2-基)硫基)乙基)喹唑啉-4(3h)-酮(ia)。
[0040][0041]
化合物ib~iw按照实施例一的方法依次合成。所合成的含1,3,4-噁二唑的喹唑啉-4(3h)-酮类衍生物(ia-iw)的结构均采用红外光谱(ir)、核磁共振谱(nmr)和高分辨质谱(hrms)进行了确证。化合物ia~iw的理化性质和光谱数据如下所示:
[0042]
3-(2-((5-(4-氯苄基)-1,3,4-噁二唑-2-基)硫基)乙基)喹唑啉-4(3h)-酮(ia):白色固体,m.p.119-121℃,产率65%;ir(kbr,cm-1
)v:1673,1607,1556,1484,1370,1321,1178,1154,1089,1020,879,776,696;
1
h nmr(400mhz,cdcl
3
)δ8.32(dd,j=8.0,1.0hz,1h,qu-5-h),8.06(s,1h,qu-2-h),7.83-7.72(m,2h,qu-7,8-2h),7.57-7.52(m,1h,qu-6-h),7.37-7.33(m,2h,ph-3,5-2h),7.26(d,j=8.6hz,2h,ph-2,6-2h),4.50(t,j=6.4hz,2h,ch
2
),4.14(s,2h,ch
2
),3.65(t,j=6.3hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ166.35,163.94,161.10,148.10,146.61,134.56,133.75,131.72,130.26,129.18,127.61,127.49,126.57,121.89,45.83,31.19,30.68;hrms(esi)m/z[m+na]
+ calcd for c
19
h
15
cln
4
nao
2
s:421.0497,found:421.0498.
[0043]
3-(2-((5-(2-甲基苄基)-1,3,4-噁二唑-2-基)硫基)乙基)喹唑啉-4(3h)-酮(ib):白色固体,m.p.101-103℃,产率58%;ir(kbr,cm-1
)v:3088,3054,1670,1613,1570,1485,1371,1325,1275,1155,1014,956,880,775;
1
h nmr(400mhz,cdcl
3
)δ8.32(dd,j=8.0,0.9hz,1h,qu-5-h),8.08(s,1h,qu-2-h),7.83-7.73(m,2h,qu-7,8-2h),7.57-7.52(m,1h,qu-6-h),7.26-7.20(m,4h,ph-3,4,5,6-4h),4.49(t,j=6.4hz,2h,ch
2
),4.17(s,2h,ch
2
),3.63(t,j=6.4hz,2h,ch
2
),2.40(s,3h,ch
3
);
13
c nmr(100mhz,cdcl
3
)δ166.61,163.63,161.09,148.08,146.66,136.71,134.54,131.83,130.77,129.78,128.02,127.59,127.47,126.58,126.55,121.90,45.87,30.67,29.59,19.61;hrms(esi)m/z[m+na]
+ calcd for c
20
h
18
n
4
nao
2
s:401.1043,found:401.1042.
[0044]
3-(2-((5-(4-氟苄基)-1,3,4-噁二唑-2-基)硫基)乙基)喹唑啉-4(3h)-酮(ic):白色固体,m.p.142-144℃,产率83%;ir(kbr,cm-1
)v:3058,2361,1668,1602,1509,1486,1364,1320,1216,1013,885,784,744,699;
1
h nmr(400mhz,cdcl
3
)δ8.32(dd,j=8.0,1.4hz,1h,qu-5-h),8.07(s,1h,qu-2-h),7.83-7.77(m,1h,qu-7-h),7.76-7.72(m,1h,qu-8-h),
7.57-7.52(m,1h,qu-6-h),7.29(dd,j=9.2,4.5hz,2h,ph-2,6-2h),7.10-7.02(m,2h,ph-3,5-2h),4.50(t,j=6.3hz,2h,ch
2
),4.14(s,2h,ch
2
),3.65(t,j=6.3hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ166.62,163.82,161.08,148.11,146.58,134.51,130.54,130.46,127.59,127.45,126.57,121.91,116.03,115.81,45.86,31.03,30.72;hrms(esi)m/z[m+na]
+ calcd for c
19
h
15
fn
4
nao
2
s:405.0792,found:405.0798.
[0045]
3-(2-((5-(4-氟苄基)-1,3,4-噁二唑-2-基)硫基)乙基)-6,7-二甲氧基喹唑啉-4(3h)-酮(id):白色固体,m.p.151-153℃,产率41%;ir(kbr,cm-1
)v:1655,1608,1503,1400,1377,1270,1222,1143,1115,1016,837,784;
1
h nmr(400mhz,cdcl
3
)δ7.98(s,1h,qu-2-h),7.62(s,1h,qu-5-h),7.32-7.26(m,2h,ph-2,6-2h),7.12(s,1h,qu-8-h),7.09-7.03(m,2h,ph-3,5-2h),4.50(t,j=6.3hz,2h,ch
2
),4.15(s,2h,ch
2
),4.02(s,6h,2ch
3
),3.64(t,j=6.3hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ166.60,163.93,163.51,161.06,160.44,155.05,149.48,145.39,144.48,130.54,130.46,129.06,129.02,116.04,115.82,115.28,107.93,105.34,56.34,45.86,31.05,30.81;hrms(esi)m/z[m+na]
+ calcd for c
21
h
19
fn
4
nao
4
s:465.1003,found:465.1004.
[0046]
3-(2-((5-(4-氟苄基)-1,3,4-噁二唑-2-基)硫基)乙基)-6-氯喹唑啉-4(3h)-酮(ie):白色固体,m.p.105-107℃,产率50%;ir(kbr,cm-1
)v:1660,1608,1510,1479,1367,1267,1221,1160,1142,1023,889,843,808;
1
h nmr(400mhz,cdcl
3
)δ8.28(d,j=2.1hz,1h,qu-5-h),8.06(s,1h,qu-2-h),7.72(dt,j=16.0,5.5hz,2h,ph-2,6-2h),7.32-7.29(m,1h,qu-7-h),7.28(s,1h,qu-8-h),7.07(t,j=8.6hz,2h,ph-3,5-2h),4.50(t,j=6.3hz,2h,ch
2
),4.16(s,2h,ch
2
),3.64(t,j=6.3hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ166.68,163.78,163.53,161.08,160.10,146.74,146.64,134.93,133.34,130.55,130.47,129.29,129.01,128.98,125.98,122.97,116.06,115.84,45.93,31.06,30.62;hrms(esi)m/z[m+na]
+ calcd for c
19
h
14
clfn
4
nao
2
s:439.0402,found:439.0401.
[0047]
3-(2-((5-((4-氯苯氧基)甲基)-1,3,4-噁二唑-2-基)硫基)乙基)喹唑啉-4(3h)-酮(if):白色固体,m.p.154-156℃,产率73%;ir(kbr,cm-1
)v:1667,1606,1493,1474,1370,1244,1169,1022,833,771,697;
1
h nmr(400mhz,cdcl
3
)δ8.32(dd,j=8.0,0.9hz,1h,qu-5-h),8.07(s,1h,qu-2-h),7.83-7.77(m,1h,qu-7-h),7.74(d,j=8.1hz,1h,qu-8-h),7.54(t,j=7.5hz,1h,qu-6-h),7.31(d,j=3.4hz,1h,ph-2-h),7.28(s,1h,ph-6-h),6.97(d,j=3.4hz,1h,ph-3-h),6.94(s,1h,ph-5-h),5.20(s,2h,ch
2
),4.51(t,j=6.3hz,2h,ch
2
),3.69(t,j=6.3hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ165.19,163.44,161.10,155.97,148.11,146.48,134.55,129.66,127.63,127.50,127.39,126.58,121.90,116.22,59.92,45.88,30.79;hrms(esi)m/z[m+na]
+ calcd for c
19
h
15
cln
4
nao
3
s:437.0446,found:437.0448.
[0048]
3-(2-((5-((4-氯苯氧基)甲基)-1,3,4-噁二唑-2-基)硫基)乙基)-6-氯喹唑啉-4(3h)-酮(ig):白色固体,m.p.153-155℃,产率78%;ir(kbr,cm-1
)v:1681,1606,1491,1472,1374,1320,1235,1168,1149,1019,837;
1
h nmr(400mhz,cdcl
3
)δ8.28(d,j=2.2hz,1h,qu-5-h),8.06(s,1h,qu-2-h),7.71(dt,j=17.3,5.5hz,2h,qu-7,8-2h),7.29(d,j=8.9hz,2h,ph-2,6-2h),6.97(t,j=6.2hz,2h,ph-3,5-2h),5.21(s,2h,ch
2
),4.51(t,j=6.4hz,2h,ch
2
),3.68(t,j=6.3hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ165.14,163.48,160.11,
155.96,146.65,134.96,133.36,129.68,129.32,127.40,125.97,122.95,116.20,59.90,45.95,30.69;hrms(esi)m/z[m+na]
+ calcd for c
19
h
14
cl
2
n
4
nao
3
s:471.0056,found:471.0063.
[0049]
3-(2-((5-((4-氯苯氧基)甲基)-1,3,4-噁二唑-2-基)硫基)乙基)-6,7-二甲氧基喹唑啉-4(3h)-酮(ih):白色固体,m.p.199-201℃,产率53%;ir(kbr,cm-1
)v:2989,1831,1674,1601,1502,1476,1407,1378,1282,1245,1216,1112,1022,970,854;
1
h nmr(400mhz,dmso-d
6
)δ8.23(s,1h,qu-2-h),7.46(s,1h,qu-5-h),7.38(d,j=8.9hz,2h,ph-2,6-2h),7.13(s,1h,qu-8-h),7.09(d,j=8.9hz,2h,ph-3,5-2h),5.32(s,2h,ch
2
),4.37(t,j=5.9hz,2h,ch
2
),3.90(s,3h,ch
3
),3.87(s,3h,ch
3
),3.68(t,j=5.9hz,2h,ch
2
);
13
c nmr(100mhz,dmso-d
6
)δ164.69,163.89,160.01,156.57,155.03,149.26,146.92,144.52,129.87,126.07,117.20,115.00,108.38,105.51,60.16,56.46,56.20,45.46,31.67;hrms(esi)m/z[m+na]
+ calcd for c
21
h
19
cln
4
nao
5
s:497.0657,found:497.0649.
[0050]
3-(2-((5-((4-氯苯氧基)甲基)-1,3,4-噁二唑-2-基)硫基)乙基)-8-甲基喹唑啉-4(3h)-酮(ii):白色固体,m.p.137-139℃,产率61%;ir(kbr,cm-1
)v:3009,1901,1674,1604,1493,1468,1367,1245,1169,1018,927,820,773,650;
1
h nmr(400mhz,cdcl
3
)δ8.17(d,j=8.0hz,1h,qu-5-h),8.08(s,1h,qu-2-h),7.64(d,j=7.3hz,1h,qu-7-h),7.43(t,j=7.7hz,1h,qu-6-h),7.33-7.27(m,2h,ph-2,6-2h),6.99-6.94(m,2h,ph-3,5-2h),5.20(s,2h,ch
2
),4.51(t,j=6.3hz,2h,ch
2
),3.70(t,j=6.3hz,2h,ch
2
),2.63(s,3h,ch
3
);
13
c nmr(100mhz,cdcl
3
)δ165.22,163.42,161.44,155.98,146.73,145.34,136.11,135.23,129.67,127.38,127.08,124.26,121.87,116.22,59.90,45.73,30.86,17.43;hrms(esi)m/z[m+na]
+ calcd for c
20
h
17
cln
4
nao
3
s:451.0602,found:451.0600.
[0051]
3-(2-((5-((4-氟苯氧基)甲基)-1,3,4-噁二唑-2-基)硫基)乙基)喹唑啉-4(3h)-酮(ij):白色固体,m.p.155-157℃,产率53%;ir(kbr,cm-1
)v:2945,1667,1606,1508,1473,1365,1249,1172,1018,974,875,826,770,694;
1
h nmr(400mhz,cdcl
3
)δ8.32(d,j=8.0hz,1h,qu-5-h),8.07(s,1h,qu-2-h),7.83-7.77(m,1h,qu-7-h),7.74(d,j=7.6hz,1h,qu-8-h),7.57-7.52(m,1h,qu-6-h),7.00(tdd,j=9.3,8.0,3.5hz,4h,ph-2,3,5,6-4h),5.19(s,2h,ch
2
),4.51(t,j=6.4hz,2h,ch
2
),3.70(t,j=6.4hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ165.11,163.63,161.10,159.31,156.92,153.53,153.51,148.11,146.49,134.54,127.62,127.49,126.58,121.90,116.33,116.29,116.21,116.09,60.42,45.87,30.78;hrms(esi)m/z[m+na]
+ calcd for c
19
h
15
fn
4
nao
3
s:421.0741,found:421.0749.
[0052]
3-(2-((5-((4-氟苯氧基)甲基)-1,3,4-噁二唑-2-基)硫基)乙基)-6,7-二甲氧基喹唑啉-4(3h)-酮(ik):白色固体,m.p.171-173℃,产率50%;ir(kbr,cm-1
)v:2983,2355,1674,1608,1503,1479,1378,1285,1216,1173,1115,1017,972,830,804;
1
h nmr(400mhz,cdcl
3
)δ8.00(s,1h,qu-2-h),7.63(s,1h,qu-5-h),7.14(s,1h,qu-8-h),7.07-6.95(m,4h,ph-2,3,5,6-4h),5.20(s,2h,ch
2
),4.51(t,j=6.3hz,2h,ch
2
),4.02(s,6h,2ch
3
),3.70(t,j=6.3hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ165.22,163.61,160.44,159.32,156.93,155.09,153.53,153.51,149.52,145.31,144.46,116.34,116.27,116.19,116.11,115.27,107.94,105.35,60.42,56.35,45.90,30.88;hrms(esi)m/z[m+na]
+ calcd for c
21
h
19
fn
4
nao
5
s:481.0952,found:481.0952.
[0053]
3-(2-((5-((4-氟苯氧基)甲基)-1,3,4-噁二唑-2-基)硫基)乙基)-6-氯喹唑啉-4(3h)-酮(il):白色固体,m.p.138-140℃,产率35%;ir(kbr,cm-1
)v:1658,1609,1508,1473,1466,1379,1325,1248,1172,1142,1026,835,825,807,645;
1
h nmr(400mhz,cdcl
3
)δ8.28(d,j=2.2hz,1h,qu-5-h),8.06(s,1h,qu-2-h),7.71(dt,j=17.2,5.5hz,2h,qu-7,8-2h),7.07-6.94(m,4h,ph-2,3,5,6-4h),5.20(s,2h,ch
2
),4.51(t,j=6.4hz,2h,ch
2
),3.68(t,j=6.4hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ165.07,163.68,160.13,159.33,156.94,153.52,153.50,146.65,134.97,133.38,129.33,125.99,122.96,116.36,116.27,116.19,116.13,60.41,45.95,30.68;hrms(esi)m/z[m+na]
+ calcd for c
19
h
14
clfn
4
nao
3
s:455.0351,found:455.0353.
[0054]
3-(2-((5-((4-氟苯氧基)甲基)-1,3,4-噁二唑-2-基)硫基)乙基))-8-甲基喹唑啉-4(3h)-酮(im):白色固体,m.p.103-105℃,产率54%;ir(kbr,cm-1
)v:3067,2918,1881,1670,1604,1509,1470,1365,1329,1168,1138,1024,931,827,808,769;
1
h nmr(400mhz,cdcl
3
)δ8.18(d,j=8.0hz,1h,qu-5-h),8.09(s,1h,qu-2-h),7.65(d,j=7.3hz,1h,qu-8-h),7.43(t,j=7.6hz,1h,qu-7-h),7.07-6.96(m,4h,ph-2,3,5,6-4h),5.19(s,2h,ch
2
),4.52(t,j=6.3hz,2h,ch
2
),3.70(t,j=6.3hz,2h,ch
2
),2.64(s,3h,ch
3
);
13
c nmr(100mhz,cdcl
3
)δ165.15,163.62,161.44,159.34,156.95,153.55,153.53,146.73,145.33,136.12,135.23,127.09,124.27,121.88,116.34,116.30,116.22,116.11,60.42,45.73,30.85,17.41;hrms(esi)m/z[m+na]
+ calcd for c
20
h
17
fn
4
nao
3
s:435.0898,found:435.0896.
[0055]
3-(2-((5-(苯氧甲基)-1,3,4-噁二唑-2-基)硫基)乙基))喹唑啉-4(3h)-酮(in):白色固体,m.p.117-119℃,产率44%;ir(kbr,cm-1
)v:1664,1607,1497,1474,1365,1249,1170,1153,1018,877,771,752,693;
1
h nmr(400mhz,cdcl
3
)δ8.33(d,j=8.0hz,1h,qu-5-h),8.10(s,1h,qu-2-h),7.80(t,j=7.3hz,1h,qu-7-h),7.76(d,j=8.1hz,1h,qu-8-h),7.55(t,j=7.4hz,1h,qu-6-h),7.35(t,j=7.7hz,2h,ph-2,6-2h),7.05(dd,j=15.2,8.0hz,3h,ph-3,4,5-3h),5.24(s,2h,ch
2
),4.51(t,j=6.4hz,2h,ch
2
),3.69(t,j=6.4hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ165.03,163.85,161.11,157.42,148.06,146.56,134.57,129.76,127.59,127.52,126.61,122.33,121.90,114.84,59.67,45.92,30.79;hrms(esi)m/z[m+na]
+ calcd for c
19
h
16
n
4
nao
3
s:403.0835,found:403.0838.
[0056]
3-(2-((5-(4-硝基苯基)-1,3,4-噁二唑-2-基)硫基)乙基))喹唑啉-4(3h)-酮(io):白色固体,m.p.206-208℃,产率56%;ir(kbr,cm-1
)v:3117,1691,1667,1607,1560,1520,1470,1343,1192,1152,1059,1006,854,777,708;
1
h nmr(400mhz,cdcl
3
)δ8.39(d,j=8.9hz,2h,ph-3,5-2h),8.32(dd,j=8.0,1.1hz,1h,qu-5-h),8.20(d,j=8.9hz,2h,ph-2,6-2h),8.11(s,1h,qu-2-h),7.82-7.76(m,1h,qu-7-h),7.73(d,j=7.5hz,1h,qu-8-h),7.53(dd,j=11.0,4.0hz,1h,qu-6-h),4.57(t,j=6.3hz,2h,ch
2
),3.78(t,j=6.3hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ164.91,164.39,161.13,149.59,148.14,146.41,134.57,128.68,127.66,127.61,127.50,126.59,124.43,121.87,45.96,30.86;hrms(esi)m/z[m+na]
+ calcd for c
18
h
13
n
5
nao
4
s:418.0581,found:418.0582.
[0057]
3-(2-((5-(4-硝基苯基)-1,3,4-噁二唑-2-基)硫基)乙基))-6-氯喹唑啉-4(3h)-酮(ip):白色固体,m.p.219-221℃,产率53%;ir(kbr,cm-1
)v:3067,1685,1601,1516,1470,1376,1350,1321,1190,1143,1059,857,831,708;
1
h nmr(400mhz,cdcl
3
)δ8.40(d,j=
8.9hz,2h,ph-3,5-2h),8.27(d,j=2.2hz,1h,qu-5-h),8.20(d,j=8.9hz,2h,ph-2,6-2h),8.10(s,1h,qu-2-h),7.71(dt,j=15.7,5.5hz,2h,qu-7,8-2h),4.57(t,j=6.3hz,2h,ch
2
),3.78(t,j=6.3hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ164.81,164.44,160.14,149.65,146.59,135.05,133.48,129.31,128.61,127.64,126.03,124.48,122.89,46.16,30.73;hrms(esi)m/z[m+na]
+ calcd for c
18
h
12
cln
5
nao
4
s:452.0191,found:452.0194.
[0058]
3-(2-((5-(3,4,5-三甲氧基苯基)-1,3,4-噁二唑-2-基)硫基)乙基))喹唑啉-4(3h)-酮(iq):白色固体,m.p.174-176℃,产率62%;ir(kbr,cm-1
)v:2946,2830,1683,1610,1556,1503,1356,1240,1125,1001,965,860,835,771,718,693;
1
h nmr(400mhz,cdcl
3
)δ8.31(dd,j=8.0,1.0hz,1h,qu-5-h),8.11(s,1h,qu-2-h),7.82-7.70(m,2h,qu-7,8-2h),7.56-7.50(m,1h,qu-6-h),7.23(s,2h,ph-2,6-2h),4.56(t,j=6.3hz,2h,ch
2
),3.96(s,6h,2ch
3
),3.94(s,3h,ch
3
),3.74(t,j=6.3hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ166.03,162.93,161.10,153.62,148.11,146.63,141.05,134.50,127.60,127.43,126.53,121.85,118.26,103.80,61.04,56.35,45.93,30.71;hrms(esi)m/z[m+na]
+ calcd for c
21
h
20
n
4
nao
5
s:463.1047,found:463.1054.
[0059]
3-(2-((5-(4-氯苯基)-1,3,4-噁二唑-2-基)硫基)乙基))喹唑啉-4(3h)-酮(ir):白色固体,m.p.135-137℃,产率46%;ir(kbr,cm-1
)v:1686,1609,1488,1473,1371,1194,1089,1071,874,834,768;
1
h nmr(400mhz,cdcl
3
)δ8.32(d,j=8.0hz,1h,qu-5-h),8.12(s,1h,qu-2-h),7.97-7.89(m,2h,ph-2,6-2h),7.82-7.69(m,2h,qu-7,8-2h),7.57-7.44(m,3h,ph-3,5-2h,qu-6-h),4.57(t,j=6.3hz,2h,ch
2
),3.74(t,j=6.3hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ165.33,163.49,161.12,148.10,146.60,138.17,134.55,129.51,127.96,127.62,127.47,126.57,121.86,121.68,45.94,30.78;hrms(esi)m/z[m+na]
+ calcd for c
18
h
13
cln
4
nao
2
s:407.0340,found:407.0345.
[0060]
3-(2-((5-(4-氯苯基)-1,3,4-噁二唑-2-基)硫基)乙基))-6,7-二甲氧基喹唑啉-4(3h)-酮(is):白色固体,m.p.105-107℃,产率62%;ir(kbr,cm-1
)v:3079,2954,2834,1677,1603,1506,1377,1288,1167,1117,1021,868,831,782,727,694;
1
h nmr(400mhz,cdcl
3
)δ8.05(s,1h,qu-2-h),7.97-7.92(m,2h,ph-2,6-2h),7.61(s,1h,qu-5-h),7.53-7.49(m,2h,ph-3,5-2h),7.13(s,1h,qu-8-h),4.55(t,j=6.3hz,2h,ch
2
),4.02(s,6h,2ch
3
),3.75(t,j=6.2hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ165.29,163.52,160.45,155.02,149.44,145.36,144.51,138.16,129.47,127.95,121.72,115.21,107.95,105.31,56.33,56.31,46.09,30.94;hrms(esi)m/z[m+na]
+ calcd for c
20
h
17
cln
4
nao
4
s:467.0551,found:467.0550.
[0061]
3-(2-((5-(4-氯苯基)-1,3,4-噁二唑-2-基)硫基)乙基))-8-甲基喹唑啉-4(3h)-酮(it):白色固体,m.p.169-171℃,产率47%;ir(kbr,cm-1
)v:1677,1606,1474,1365,1189,1136,1091,833,768,728;
1
h nmr(400mhz,cdcl
3
)δ8.18(d,j=8.0hz,1h,qu-5-h),8.13(s,1h,qu-2-h),7.98-7.92(m,2h,ph-2,6-2h),7.64(d,j=7.3hz,1h,qu-7-h),7.53-7.49(m,2h,ph-3,5-2h),7.42(t,j=7.7hz,1h,qu-6-h),4.57(t,j=6.2hz,2h,ch
2
),3.75(t,j=6.2hz,2h,ch
2
),2.62(s,3h,ch
3
);
13
c nmr(100mhz,cdcl
3
)δ165.32,163.50,161.46,146.73,145.39,138.18,136.10,135.19,129.51,127.96,127.04,124.27,121.85,121.73,45.84,30.88,17.43;hrms(esi)m/z[m+na]
+ calcd for c
19
h
15
cln
4
nao
2
s:421.0497,found:
421.0497.
[0062]
3-(2-((5-(呋喃-2-基)-1,3,4-噁二唑-2-基)硫基)乙基))喹唑啉-4(3h)-酮(iu):白色固体,m.p.114-116℃,产率48%;ir(kbr,cm-1
)v:3096,1681,1610,1471,1371,1292,1209,1155,1088,1018,898,772,750,698;
1
h nmr(400mhz,cdcl
3
)δ8.32(dd,j=8.0,1.0hz,1h,qu-5-h),8.10(s,1h,qu-2-h),7.82-7.71(m,2h,qu-7,8-2h),7.65(dd,j=1.7,0.6hz,1h,furan-5-h),7.56-7.50(m,1h,qu-6-h),7.15-7.12(m,1h,furan-4-h),6.61(dd,j=3.5,1.8hz,1h,furan-3-h),4.54(t,j=6.3hz,2h,ch
2
),3.73(t,j=6.3hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ162.68,161.11,158.85,148.14,146.58,145.88,138.76,134.48,127.62,127.41,126.56,121.89,114.29,112.19,45.93,30.88;hrms(esi)m/z[m+na]
+ calcd for c
16
h
12
n
4
nao
3
s:363.0522,found:363.0524.
[0063]
3-(2-((5-(呋喃-2-基)-1,3,4-噁二唑-2-基)硫基)乙基))-6-氯喹唑啉-4(3h)-酮(iv):白色固体,m.p.150-152℃,产率48%;ir(kbr,cm-1
)v:1666,1607,1468,1364,1326,1199,1162,1148,1090,1016,899,854,749;
1
h nmr(400mhz,cdcl
3
)δ8.28(d,j=2.1hz,1h,qu-5-h),8.10(s,1h,qu-2-h),7.70(ddd,j=12.1,6.0,1.6hz,3h,qu-7,8-2h,furan-5-h),7.15(d,j=3.5hz,1h,furan-3-h),6.62(dd,j=3.5,1.7hz,1h,furan-4-h),4.55(t,j=6.3hz,2h,ch
2
),3.72(t,j=6.3hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ162.60,160.15,158.92,146.76,146.64,145.96,138.71,134.96,133.35,129.31,125.99,122.94,114.39,112.25,46.10,30.76;hrms(esi)m/z[m+na]
+ calcd for c
16
h
11
cln
4
nao
3
s:397.0133,found:397.0134.
[0064]
3-(2-((5-(4-氯苯基)-1,3,4-噁二唑-2-基)硫基)乙基))-6-氯喹唑啉-4(3h)-酮(iw):白色固体,m.p.179-181℃,产率55%;ir(kbr,cm-1
)v:1676,1606,1469,1370,1322,1194,1145,1084,1005,830,725,640;
1
h nmr(400mhz,cdcl
3
)δ8.27(d,j=2.1hz,1h,qu-5-h),8.10(s,1h,qu-2-h),7.96-7.91(m,2h,ph-2,6-2h),7.70(dt,j=15.4,5.5hz,2h,qu-7,8-2h),7.54-7.48(m,2h,ph-3,5-2h),4.56(t,j=6.3hz,2h,ch
2
),3.73(t,j=6.3hz,2h,ch
2
);
13
c nmr(100mhz,cdcl
3
)δ165.38,163.37,160.12,146.71,146.62,138.27,134.94,133.35,129.53,129.30,127.96,125.98,122.90,121.64,46.13,30.70;hrms(esi)m/z[m+na]
+ calcd for c
18
h
12
cl
2
n
4
nao
2
s:440.9950,found:440.9951.
[0065]
实施例二:含1,3,4-噁二唑的喹唑啉-4(3h)-酮类衍生物(ia-iw)的抑菌活性
[0066]
测试方法:
[0067]
采用浊度法测试了含1,3,4-噁二唑的喹唑啉-4(3h)-酮类衍生物(ia-iw)对水稻白叶枯病菌(xanthomonas oryzae pv.oryzae,xoo)的抑制活性,具体操作步骤如下:1)于1000ml灭菌蒸馏水中加入蛋白胨5.0g、酵母粉1.0g、葡萄糖10.0g、牛肉膏3.0g,调节ph至中性(7.2
±
0.2);2)向每支试管内移取第一步中的溶液4.0ml,在121℃灭菌20min后待用;3)称取4mg待测样品于离心管中,以320μl dmso溶解,移取80μl到离心管中,加入4ml的0.1%tween-20溶液。设噻菌铜或叶枯唑作对照药剂,dmso作空白对照;4)从离心管内移取1ml溶液到3支第二步中的试管内;5)取96孔板,测空白od值,排除od值大于0.05的孔,向每个可用孔中加入200μl第4步中试管内溶液,测od值,最后向每支试管中接入40μl活化后的水稻白叶枯菌种,在30℃、180rpm恒温摇床中振荡培养24~48h,期间测试试管内溶液od值以跟踪细菌生长状态,培养结束后在试管中取200μl溶液,测od值;6)化合物对细菌抑制率计算公
式如下:抑菌率=(对照菌液od值-样品菌液od值)/对照菌液od值
×
100%,其中,菌液od值=含菌培养基od值-无菌培养基od值。
[0068]
采用菌丝生长速度法测定了一种含1,3,4-噁二唑的喹唑啉-4(3h)-酮类衍生物(ia-iw)对小麦赤霉病菌(fusarium graminearum,fg)和水稻纹枯病菌(rhizoctonia solani,rs)的抑制活性,具体操作步骤如下:1)称取一定量的化合物溶于一定体积的dmso中,得到一定浓度的母液;2)量取100μl母液加入到45ml已灭菌的土豆琼脂培养基中,震荡均匀;3)将上述培养基均匀倒入3皿直径为9厘米的培养皿中,待其凝固后,将直径为5毫米的菌饼转接至培养皿中心;4)将上述培养皿在25
±
1℃条件下,培养至菌落直接约为7.0至7.5厘米后,测定菌落直径,计算各药剂的抑制率。5)化合物对真菌抑制率计算公式如下:抑菌率=(空白对照菌落直径-测试药剂菌落直径)
÷
(空白对照菌落直径-5mm)
×
100%。
[0069]
化合物ia-iw对水稻白叶枯病菌(xoo)、水稻纹枯病菌(rs)和小麦赤霉病菌(fg)的抑制活性测定结果如表2所示。
[0070]
表2 化合物ia-iw对3种病菌的抑制活性
a
(50μg/ml)
[0071][0072]
a
三次重复,取其平均值。
[0073]
化合物(ia-iw)在50μg/ml时对水稻白叶枯菌(xoo)的抑制活性测试结果(见表2)表明,大部分化合物对水稻白叶枯菌具有明显的抑制活性。其中,化合物ie-ig和ii-iw对水稻白叶枯菌的抑制率介于分别为32.15%-54.11%,优于对照药剂噻菌铜(31.64%)。
[0074]
化合物(ia-iw)在50μg/ml时对水稻纹枯病菌(rs)和小麦赤霉病菌(fg)的抑制活性测试结果(见表2)表明,化合物ia、ib、ic、ie、ii、im、ir和iu对水稻纹枯病菌的抑制率分别为70.61、58.26、52.88、59.95、65.45、70.89、73.47和73.74%,优于对照药剂恶霉灵(33.90%)。化合物ii、im和ir对小麦赤霉病菌的抑制率分别为52.17、52.42和59.42%,优于对照药剂恶霉灵(46.86%)。
[0075]
以上所述,仅是本发明的较佳实施案例而已,并非对本发明作任何形式上的限制,
任何未脱离本发明技术方案内容,依据本发明的技术实质对以上实施例所做的任何简单修改、等同变化与修饰,均属于本发明技术方案的范畴。
起点商标作为专业知识产权交易平台,可以帮助大家解决很多问题,如果大家想要了解更多知产交易信息请点击 【在线咨询】或添加微信 【19522093243】 与客服一对一沟通,为大家解决相关问题。
与客服一对一沟通,为大家解决相关问题。
此文章来源于网络,如有侵权,请联系删除
相关标签: esi
 热门咨询
热门咨询

tips


 商标分类
商标分类  商标转让
商标转让 



